Two-year clinical evaluation of Class I composite resin restorations using three adhesive systems: A double-blind randomized clinical trial
Abstract
Aim: The purpose of this randomized split-mouth clinical study was to assess the effect of three adhesive systems on the 2-year clinical success of Class I composite resin restorations.
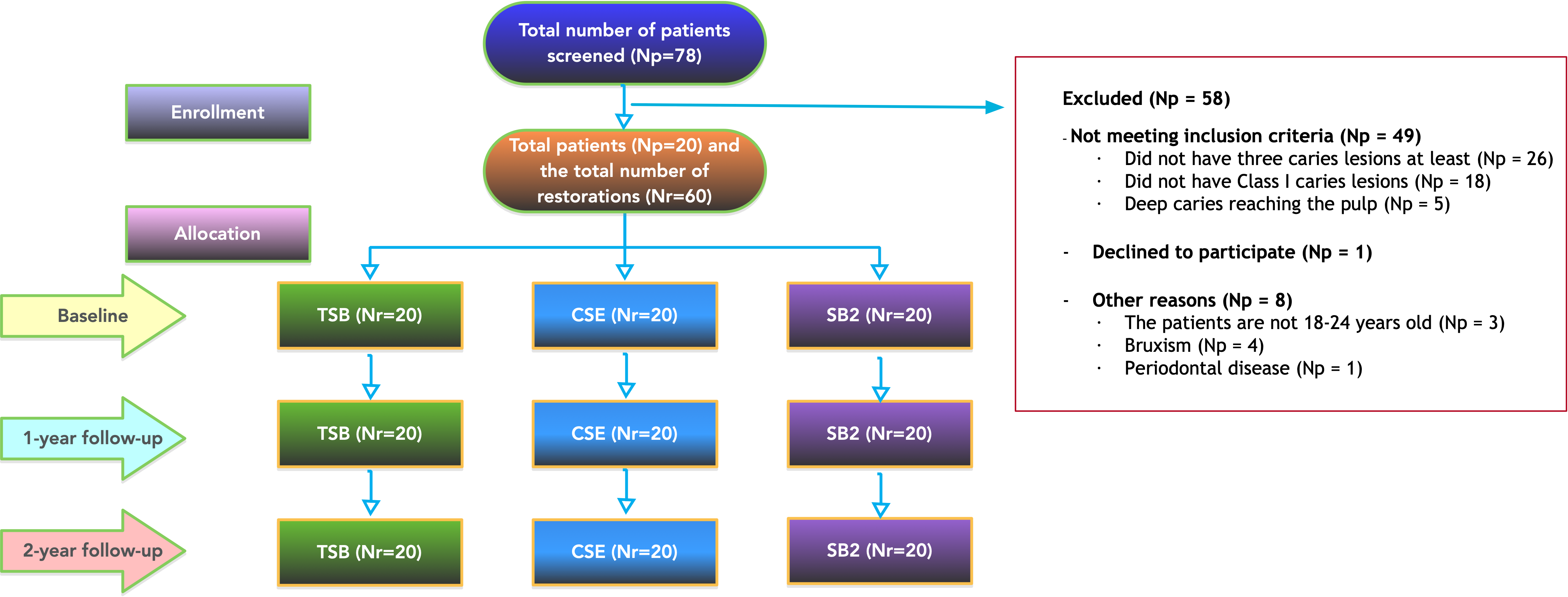
Methodology: In the treatment of the Class I carious lesions of 20 participants aged 18–24 years with at least three similar carious lesions, three adhesives—Clearfil SE Bond (CSE; Kuraray, Osaka, Japan), Single Bond 2 (SB2; 3M ESPE, St. Paul, MN, USA), and Tri-S Bond (TSB; Kuraray, Osaka, Japan)—and a Filtek Z550 nanohybrid composite resin (3M ESPE, St. Paul, MN, USA) were cured. The baseline and 2-year results of the restorations were assessed according to the World Dental Federation (FDI) and the United States Public Health Service (USPHS) criteria. The chi-square test was used to analyze the data obtained.
Results: There was no loss of restoration in any group at 2 years. No significant differences were observed in any criteria (marginal staining, fracture retention, secondary caries, and postoperative sensitivity) evaluated except marginal adaptation, in accordance with FDI and USPHS criteria (p > 0.05). At 2 years, SB2 showed the best marginal adaptation, followed by CSE and TBS. There was a statistically significant difference between SB2 and TSB (p ˂ 0.05).
Conclusion: All three adhesive systems can be used successfully in the restoration of Class I carious lesions.
How to cite this article:
Çakır Kılınç NN, Demirbuğa S. Two-year clinical evaluation of Class I composite resin restorations using three adhesive systems: A double-blind randomized clinical trial. Int Dent Res 2023;13(2):67-74. https://doi.org/10.5577/idr.2023.vol13.no2.4
Linguistic Revision: The English in this manuscript has been checked by at least two professional editors, both native speakers of English.
Full text article
Authors
Copyright © 2023 International Dental Research

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.